Beyond Fermentation
CKD BiO, spun off in 2001 as a subsidiary of the esteemed CKD Group founded in 1941, began its journey in active pharmaceutical ingredients (APIs). Today, we are exploring innovative avenues beyond fermentation, notably in our microbiome business, as we continue to expand our horizons and uphold our tradition of excellence.
We are expanding from APIs to botulinum toxin, probiotics, and microbiome businesses based on our fermentation technology.
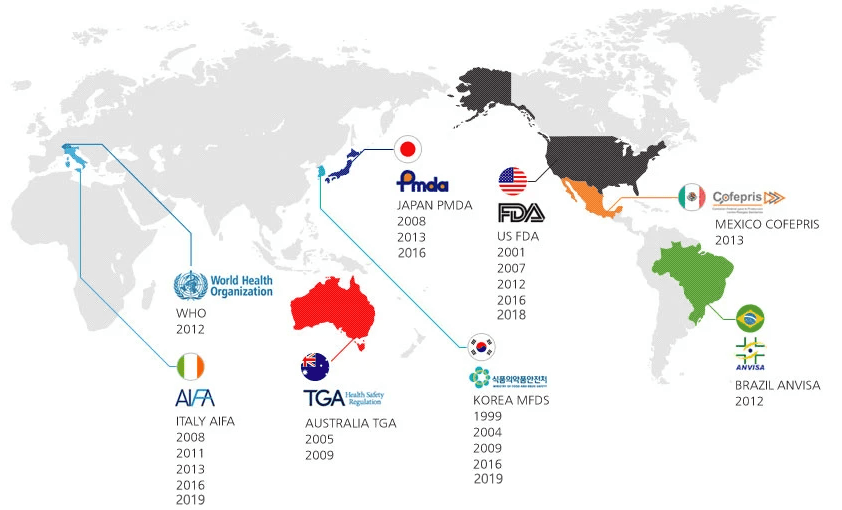
GMP Certification
We are always following & complying with the latest global GMP standard.

Agency
South Korea (MFDS)
USA (FDA)
Australia (TGA)
Indication
Skin disease
Lupus disease
Reproductive tract infections
Inflammatory bowel disease
Rheumatoid arthritis
Cachexia
Non-alcoholic fatty hepatitis
Alzheimer's disease
New CDMO Expertise
Strict Anaerobic Microbiome CDMO
EV-based Therapy CDMO
eLBP CDMO