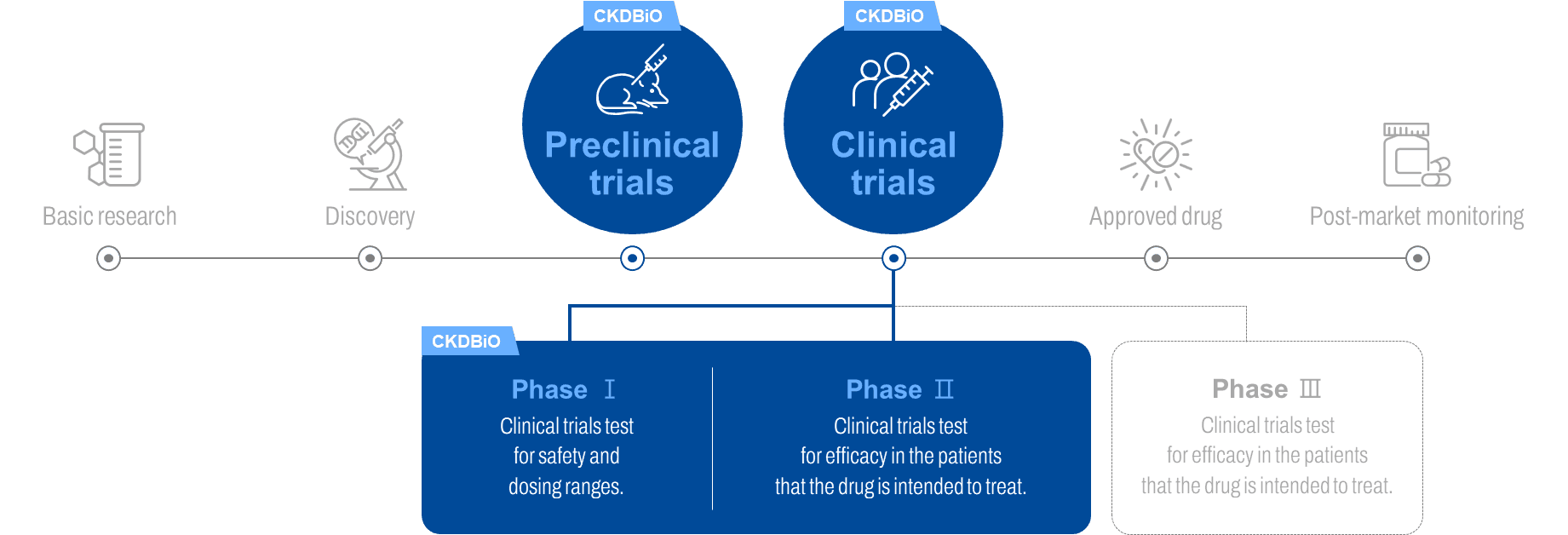
Early Clinical Phase Specialized CDMO Service
CKD BiO is providing comprehensive array of services, spanning from initial process development to GMP manufacturing tailored for Phase I & II clinical trials.
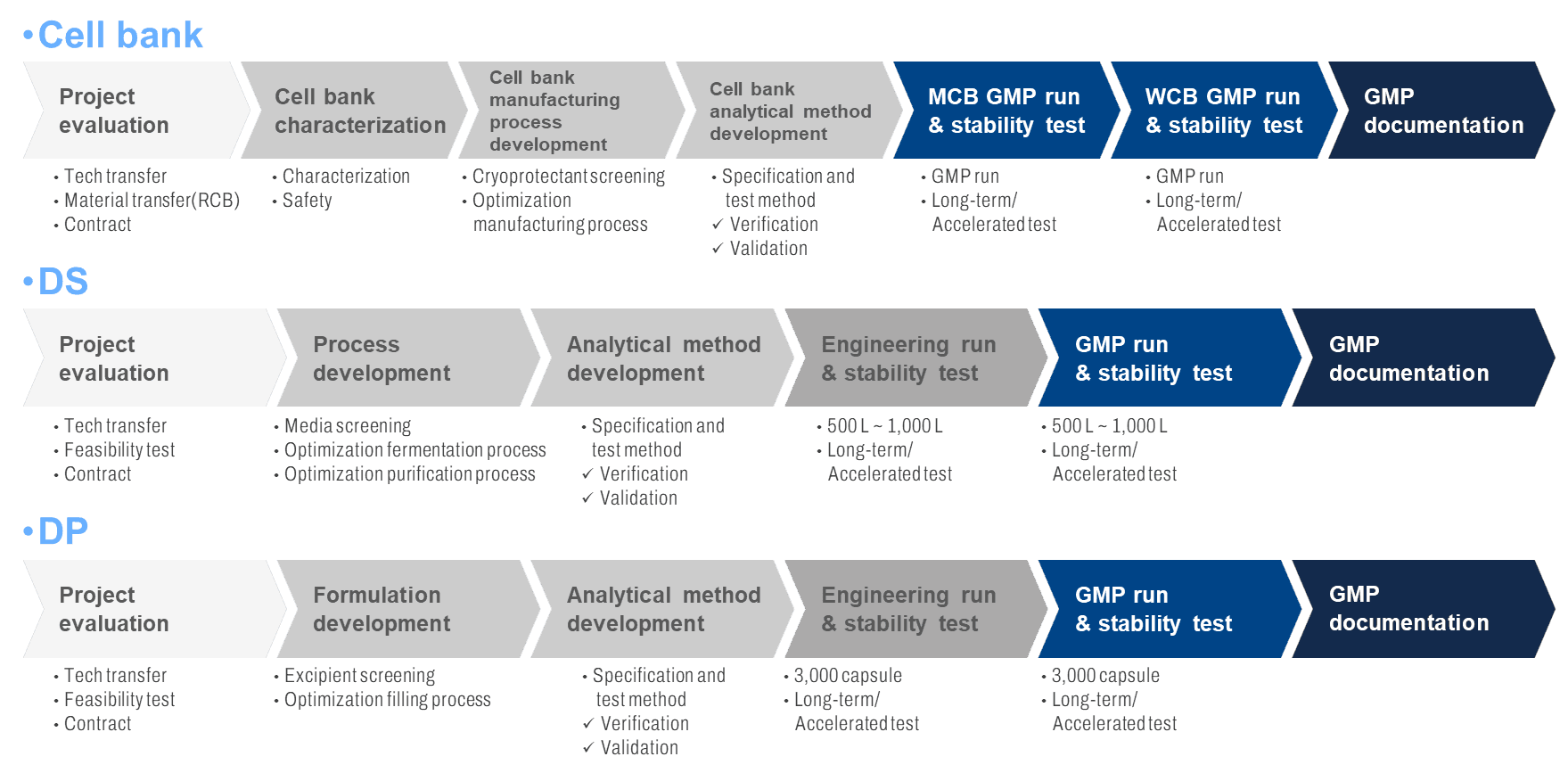
One-stop Platform of CDMO Service
A comprehensive one-stop biopharmaceutical service offering customized, GMP-compliant manufacturing solutions from cell banking through final drug product, with integrated quality tracking and regulatory documentation